Qing Chen, M.D., Ph.D.
-
Assistant Professor, Immunology, Microenvironment and Metastasis Program, Ellen and Ronald Caplan Cancer Center
-
Scientific Director, Imaging Facility
Chen focuses on the molecular mechanisms of brain metastasis originating from primary tumors like breast cancer, and the interplay between cancer cells and the stromal cells that populate the brain microenvironment.
After obtaining her M.D. degree in China, Chen decided to switch her career path from clinical medicine to basic research with a singular goal: to gain a better understanding of cancer biology so we can one day provide effective treatments and perhaps even cures. She came to the United States to pursue her graduate research career at Roswell Park Cancer Institute. After obtaining her Ph.D., she joined the group of Joan Massagué, Ph.D., at Memorial Sloan-Kettering Cancer Center as a postdoctoral fellow. In October 2015, Chen joined the Tumor Microenvironment and Metastasis Program at the Wistar Institute.
The Chen Laboratory

The Chen Laboratory
The Chen laboratory studies cancer metastasis, the spread of cancer from the primary tumor site to distal organs, which is the cause of 90% of deaths from cancer. The brain is one of the common metastasis locations in breast cancer. Therapeutic strategies, including novel chemotherapies and targeted inhibitors, have limited efficacy in brain metastatic lesions. As a consequence, the incidence of brain metastases has increased in recent years, especially in triple-negative and HER2-positive breast cancer patients. Metastatic outgrowth requires the complex interplay between cancer cells and the microenvironment in distal organs.
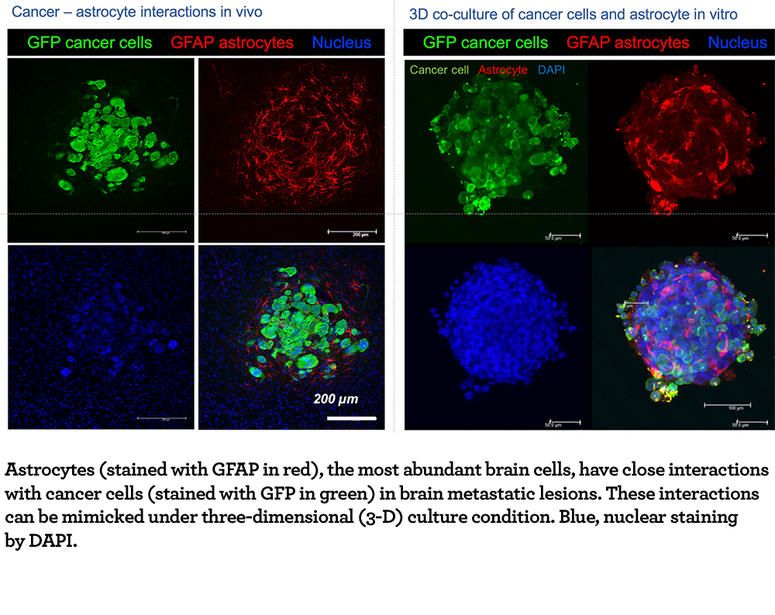
Our lab focuses on this crosstalk to reveal mechanisms that mediate cancer survival/growth in distal organs. We are particularly interested in the metastatic outgrowth in the unique brain microenvironment. For example, astrocytes (stromal cells that only exist in the brain) densely infiltrate into brain metastatic lesions. Notably, astrocytes have dual functions — killing and protecting, with respect to the invaded cancer cells. We are taking a multidisciplinary approach, spanning molecular and biochemical analyses combined with sophisticated in vivo imaging, with the goal of dissecting the dynamic cancer cell-astrocyte interactions both temporally and spatially. The mechanistic insights into the metastatic process will facilitate the development of more effective therapies.
-
Postdoctoral Fellows
Weili Ma, Ph.D.
Maria Cecilia Oliveira Ferreira Nunes, Ph.D.
Yongkang Zou, Ph.D.
-
Postdoctoral fellow positions are available in the Chen laboratory with a research focus on cancer metastasis, particularly in the brain.
Candidates should have recently received, or be close to obtaining their Ph.D. degree (or equivalent) and have a strong background in one or more of the following disciplines: cancer biology, neurobiology, immunology, small animal surgeries, and microscopy.
Interested applicants are invited to submit a cover letter, CV and a list of three references to qichen@Wistar.org
Research
Metastasis presents a major threat to the lives of cancer patients. Brain metastasis is becoming a significant clinical problem and its incidence is rising. This is largely attributable to the fact that current therapies, which are effective in controlling extracranial metastasis and prolong patient survival, are ineffective in controlling metastatic disease of the brain. Our goal is to elucidate the underlying mechanisms that mediate metastasis and therapeutic resistance in the brain.
-
Postdoctoral Fellows
Weili Ma, Ph.D.
Maria Cecilia Oliveira Ferreira Nunes, Ph.D.
Yongkang Zou, Ph.D.
-
Postdoctoral fellow positions are available in the Chen laboratory with a research focus on cancer metastasis, particularly in the brain.
Candidates should have recently received, or be close to obtaining their Ph.D. degree (or equivalent) and have a strong background in one or more of the following disciplines: cancer biology, neurobiology, immunology, small animal surgeries, and microscopy.
Interested applicants are invited to submit a cover letter, CV and a list of three references to qichen@Wistar.org
Emerging evidence indicates that metastatic outgrowth at distal organs requires the complex interplay between cancer cells and stromal cells, a process commonly referred to as “seed and soil hypothesis”. The “soil”, specifically the brain, is composed of distinct cell types: functional neurons and supporting glial cells. Not only does this unique brain microenvironment determine the outgrowth of metastatic cancer cells, but it also contributes to therapy resistance. Meanwhile, the “seeds”, or the invaded cancer cells, modify the surrounding brain stromal cells. Thus, the lab is applying innovative approaches to dissect the crosstalk between cancer cells and the brain microenvironment at various stages of metastatic development to identify potential targets to prevent and/or treat brain metastasis.
Chen Lab in the News
Selected Publications
Polyunsaturated Fatty Acids from Astrocytes Activate PPAR Gamma Signaling in Cancer Cells to Promote Brain Metastasis.
Zou, Y., Watters, A., Cheng, N., Perry, C.E., Xu, K., Alicea, G.M., Parris, JL,D., Baraban, E., Ray, P., Nayak, A., Xu, X., Herlyn, M., Murphy, M.E., Weeraratna, A.T., Schug, Z.T., Chen, Q. “Polyunsaturated Fatty Acids from Astrocytes Activate PPAR Gamma Signaling in Cancer Cells to Promote Brain Metastasis” Cancer Discov. 2019 Oct 2. pii: CD-19-0270. doi: 10.1158/2159-8290.CD-19-0270.
Type I interferon response in astrocytes promotes brain metastasis by enhancing monocytic myeloid cell recruitment
Ma W, Oliveira-Nunes MC, Xu K, Kossenkov A, Reiner BC, Crist RC, Hayden J, Chen Q. Type I interferon response in astrocytes promotes brain metastasis by enhancing monocytic myeloid cell recruitment. Nat Commun. 2023 May 6;14(1):2632. doi: 10.1038/s41467-023-38252-8. PMID: 37149684; PMCID: PMC10163863.
Mutant p53 controls tumor metabolism and metastasis by regulating PGC-1α.
Basu, S., Gnanapradeepan, K., Barnoud, T., Kung, C.P., Tavecchio, M., Scott, J., Watters, A., Chen, Q., Kossenkov, A.V., Murphy, M.E. “Mutant p53 controls tumor metabolism and metastasis by regulating PGC-1α.” Genes Dev. 2018 Feb 1;32(3-4):230-243. doi: 10.1101/gad.309062.117. Epub 2018 Feb 20.
Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer.
Chen, Q., Boire, A., Jin, X., Valiente, M., Er, E.E., Lopez-Soto, A., Jacob, L., Patwa, R., Shah, H., Xu, K., et al. “Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer.” Nature. 2016 May 26;533(7604):493-498. doi: 10.1038/nature18268. Epub 2016 May 18.
Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs.
Chen, Q., Zhang, X.H., Massagué, J. “Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs.” Cancer Cell. 2011 Oct 18;20(4):538-49. doi: 10.1016/j.ccr.2011.08.025.


