Luis J. Montaner, D.V.M., D.Phil
-
Executive Vice President
-
Director, HIV Cure and Viral Diseases Center
-
Herbert Kean, M.D., Family Professor
-
Associate Director for Shared Resources, Ellen and Ronald Caplan Cancer Center
-
Genome Regulation and Cell Signaling Program, Ellen and Ronald Caplan Cancer Center
-
Scientific Director, Humanized Models of Disease Facility
- Scientific Director, Flow Cytometry Facility
- Scientific Director, Biomedical Research Support Facility
Montaner studies the mechanisms of disease in HIV-1 infection, cancer, COVID-19, and emerging viral infections (monkeypox), exploring new strategies to boost the natural function of the immune system in order to combat viral-associated disease or cancer progression.
Montaner obtained his D.V.M., Veterinary Medicine from Kansas State University in 1989 and his D.Phil. in Experimental Pathology from University of Oxford, U.K., in 1995. He joined The Wistar Institute in 1995 as an assistant professor and was promoted to professor in 2007. Montaner was named the Herbert Kean, M.D., Family Endowed Chair Professorship in 2015.
The Montaner Laboratory

The Montaner Laboratory
At Wistar, the Montaner laboratory focuses on immune system-based research using laboratory models of virus infection, animal models of infection and/or cancer, and clinical cohort studies to provide a clinic-to-bench research program that informs new strategies to combat HIV and or cancer. The Montaner lab is also a leading center of a Martin Delaney Collaboratory focused on HIV cure-directed research (see beat-hiv.org). Patient- and animal-based collaborative studies extend from Philadelphia across the United States and Puerto Rico, Mexico, Europe, South America, Southern Africa, and Vietnam. Current research focuses on:
- Identifying new strategies to reverse mechanisms of immunodeficiency caused by viral infection and/or cancer processes via testing new immune-enhancing strategies in patient-based studies (specimens, clinical trials), and animal models (humanized mice models, non-human primates).
- Exploring new ways to augment HIV-1 control beyond current therapies in order to achieve durable remission and/or permanent control of infection without the need for continued antiretroviral therapy.
- Understanding the role of targeting myeloid cells in cancer progression.
- Determining the impact of substance use disorder therapy on immune functionality and HIV reservoir retention in opioid-dependent persons living with HIV.
- Determining the impact of COVID-19 infection and/or vaccination on immune activation and HIV reservoirs in persons living with HIV.
- Antiviral discovery strategies based on natural products and small molecule lead optimization.
Watch this video to learn more about HIV cure research.
-
Research Assistant Professor
Ian Tietjen, Ph.D.
-
Senior Staff Scientists
Livio Azzoni, M.D., Ph.D.
Emmanouil Papasavvas, Ph.D.
Costin Tomescu, Ph.D.
Zhe Yuan, Ph.D. -
Staff Scientist
Gwendolyn Cramer, Ph.D.
-
Postdoctoral Fellows
Avishek Bhuniya, Ph.D. <br> Khumoekae Richard, Ph.D. <br> Dmitry Zhigarev, Ph.D.
-
Research Assistants
Maria de Grecia Cauti Mendoza
Yong Chen
Matthew Fair
Adiana Ochoa Ortiz
Emery Register
Brian Ross
Paridhima Sharma
Qixun Sun
Xiao Sun
Guorui Zu -
Clinical Coordinator
Ken Lynn, R.N.
-
Clinical Research Assistant
Bashir Fadulalmola <br> Ola Mohamed
-
BSL-2+ Laboratory Manager
Jessicamarie Morris
-
Associate Director, HIV Program
Beth Peterson
-
Administrative Coordinator
Chelsea Anderson
-
Postdoctoral fellow positions are available in the Montaner laboratory, with a focus on cancer and immunotherapy system-based research through a combination of both murine-based models and human translational studies. The preferred candidate is a recent Ph.D. or equivalent with a strong background in immunology, animal models, and molecular/cellular biology. Experience with molecular screening, bioinformatics, microscopy, and flow cytometry is a plus. Individuals eligible for National Research Service Award (NRSA) funding are highly desirable. Motivated candidates are encouraged to submit their CV and references to: montanerlab@wistar.org.
Research
The Montaner lab directs several international teams and advances basic translational research focused on immunology, infectious disease, and cancer. The lab has expertise on human immunology (innate response), HIV cure-directed efforts, cancer immunotherapy, and clinical trials.
In 2021, the BEAT-HIV Collaboratory received a second, five-year, $29.15 million Martin Delaney Collaboratory (MDC) award and was joined by a third principal investigator Robert F. Siliciano, M.D., Ph.D., from Johns Hopkins University in addition to co-principal investigators Luis J. Montaner, D.V.M., D.Phil., from Wistar, and James L. Riley, Ph.D., from the University of Pennsylvania. The new award is one of 10 grants funded in 2021 by the National Institutes of Health’s (NIH) “Martin Delaney Collaboratories to Cure HIV” initiative to a highly-select group of U.S.-led teams charged with advancing global efforts to develop a cure for HIV. Building on the success of the initial award made in 2016, when the NIH granted nearly $23 million to the BEAT-HIV Delaney Collaboratory to Cure HIV-1 Infection by Combination Immunotherapy. While the initial cycle from 2016-2021 funded the BEAT2 clinical trial, the most recent cycle of funding focuses more on basic science and did not include clinical trial support.
The BEAT-HIV Delaney Collaboratory is a partnership of more than 85 leading HIV investigators, from academia to industry partners working with community-based organization Philadelphia FIGHT, to test combinations of several novel immunotherapies under new preclinical research. The new cycle of BEAT-HIV funding established three research goals, based on goals outlined in the NIH program announcement:
- Understand the basic mechanisms underlying persistence of the viral reservoir during ART, what cell populations contribute to rebound after treatment interruption, and the role played by host-related factors.
- Develop strategies to achieve durable suppression of HIV replication in the absence of ART. Capitalizing on advances in clinical research on bNAbs, BEAT-HIV researchers will test synthetic DNA technology to better deliver the genetic blueprint for the body to make different specific bNAbs simultaneously. An additional approach will be to boost natural killer and T cell responses to achieve long-term viral suppression. Eventually, the two strategies will be combined to maximize long-term control potential.
- Develop new approaches to eradicate the HIV reservoir. Strategies to be tested include novel drugs able to reactivate latent HIV hiding in the immune cells, combined with CAR-T cell approaches designed to change a person’s killer T cells to make them able to find infected cells more efficiently. In addition, researchers will apply a technology called mRNA-LNP to make cells resistant to HIV. The ultimate goal is to identify which approaches have the best potential and test them in combination to achieve complete HIV eradication.
BEAT-HIV2 CLINICAL STUDIES – COMPLETE
We completed enrollment in each HIV cure-directed clinical trial funded under the BEAT-HIV umbrella from 2016-2021.
The first study was to determine if treatment with pegylated interferon alpha 2b (peg-IFN-α2b) together with neutralizing antibodies 3BNC117+10-1074 will result in a reduction of viral rebound and reduction in the amount of latent HIV DNA in peripheral blood cells and tissues of individuals with chronic HIV infection upon an antiretroviral treatment (ART) interruption. By measuring the changes in viral rebound after ART interruption as a surrogate measure of the latent reservoir and immune control, the study will establish if this combined immunotherapy strategy should be considered as a component of future viral eradication strategies. Visit ClinicalTrials.gov under NCT03588715 for more information. Visit ClinicalTrials.gov under NCT03588715 for more information. Data analysis is underway.
The second study tested a clinical strategy that combined two gene therapy vectors to genetically modify T cells purified from study participants using the chimeric antigen receptor (CAR) technology to make these cells highly specific in recognizing HIV-infected cells. In addition, these T cells will be made HIV-resistant by using Zinc-finger Nucleases (ZFNs) that target CCR5, an HIV entry molecule. As a result of these genetic modifications, immune cells will be rendered specific in their killing capacity while also resistant against HIV infection, which is expected to enhance their intrinsic ability to clear HIV-infected cells and result in durable viral suppression after suspension of the antiretroviral therapy. To learn more about this clinical trial, visit ClinicalTrials.gov (NCT03617198). Data analysis is underway.
HOME-BASED VIRAL LOAD TESTING DEVICE – ENROLLMENT COMPLETE
BEAT-HIV investigators partnered with Merck, Inc. and Tasso, Inc. to assess the reliability and acceptability of a home-based viral load testing device. The micro-blood collection device was previously tested concurrently with participants enrolled in each of the two BEAT-HIV clinical trials described above.
HIV cure-directed clinical trials often include an analytic treatment interruption (ATI, learn more about that here). ATIs require frequent clinic visits to monitor participants’ viral load to keep it within study safety guidelines. The COVID epidemic changed the research landscape, including a stated desire among study participants and community advocates to reduce the number of study visits (and potential exposure to COVID-19 during transit and at the clinic).
The device being tested could potentially reduce the number of required clinic visits for blood collection, but only if the home-based viral load device test works as well as standard lab-based viral load testing that can now only be done at the clinic. Just as important is to determine how people living with HIV feel about the device and the process for returning it to the lab by mail or courier, how comfortable they are with using the home-based device, and if they have any other concerns.
The home-based viral load testing device is currently being offered by invitation to participants resuming antiretroviral therapy. At this time, the home-based viral load testing device is available within an experimental framework to determine if: 1) the device is acceptable to participants and 2) if the device provides results that are comparable to those available with traditional clinic- or lab-based viral load testing.
OPIOIDS AND HIV: PHILADELPHIA-BASED STUDY TO DEFINE HOST FACTORS DRIVING HIV INFECTION AND IMPACTING OPTIMAL MANAGEMENT OF OPIOID USE DISORDER
Pennsylvania is at the center of the opioid and HIV epidemic in the U.S., leading in both new HIV infections associated with persons who inject drugs (PWIDs) and in overdose deaths due to lack of optimal management of opioid drug addiction. In 2019, the federal government identified Philadelphia County as one of 48 counties responsible for >50% of new HIV diagnoses in the U.S. New infections in Philadelphia have been linked to an increase in HIV transmission from PWIDs.
Pennsylvania also ranks fourth nationwide for deaths due to injection-drug overdose. Management of opioid addiction in persons living with HIV who are already in care for their HIV disease can be achieved by use of medications for opioid use disorder (MOUDs), including methadone, buprenorphine, or naltrexone. Even though these medications can impact immune activation on their own, what remains unknown is how the choice of methadone, buprenorphine, or naltrexone could affect the immune recovery and therefore, the future health of persons taking medications for their HIV disease and MOUD.
The Montaner lab and collaborators in Prevention Point Philadelphia, University of Pennsylvania, and Philadelphia FIGHT are investigating the host factors driving new HIV infections in persons who inject drugs and the impact of MOUDs on immune recovery after HIV suppression by antiretroviral therapy (ART).
PHILADELPHIA MECHANISTIC STUDY – ENROLLMENT COMPLETE
We previously established clinical access to populations of interest in Philadelphia and engaged this clinical infrastructure (mobile units and multi-city clinical sites) to collect substance use/behavior (questionnaires) and biological data (blood samples) from people who inject drugs (PWIDs) who are at high risk of HIV infection, as well as PWIDs and who are living with HIV who are taking medications for opioid use disorder. In collaboration with University of Pennsylvania, Jonathan Lax Treatment Center, the Icahn School of Medicine at Mount Sinai, and Prevention Point Philadelphia among others, we conducted a mechanistic study to determine the levels of inflammation and innate immune activation in depressed and non-depressed PWIDs, and if levels of immune activation can impact HIV susceptibility ex vivo. We will also define levels of immune activation associated with comorbidities (if elevated) in PWIDs living with HIV and who are stably suppressed under ART and taking either methadone, buprenorphine, or naltrexone to evaluate the impact of long-term opioid receptor stimulation or blockage with MOUDs on immune reconstitution in persons living with HIV. The long-term impact of this study is to investigate novel factors that can be targeted for HIV prevention in PWIDs and/or how best to manage opioid drug addiction in persons living with HIV to ensure the best immune recovery after HIV therapy able to reduce added comorbidities in the future while increasing overall survival.
AMOHI CLINICAL TRIAL (VIETNAM)
NIH-funded clinical trial to investigate the link between retention of chronic immune activation in HIV-1-infected opioid users receiving medication for opioid use disorder (MOUD) combined with antiretroviral therapy (ART) and starting on methadone maintenance, when compared to naltrexone or buprenorphine.
The Montaner lab leads an international team composed of investigators from the U.S., Vietnam, and France, in collaboration with the Vietnam Ministry of Health, University of Pennsylvania, IMEA (a French-led initiative to expand access to HIV/hepatitis prevention and treatment services), the Pasteur Institute, and industry partners Alkermes, plc and Rusan. The goal of this three-arm randomized clinical trial is to evaluate the impact of long-term opioid receptor stimulation or blockage with MOUDs on immune reconstitution in people living with HIV who inject drugs and who are initiating ART. Preliminary data suggest that chronic opioid receptor engagement by an opioid receptor agonist while on ART may result in increased immune activation and inflammation associated with increased levels of persistent HIV, when compared to a full opioid receptor antagonist. To verify this hypothesis, the study will assess recovery outcomes and adherence to therapy 48 weeks after initiation of ART in 225 participants with OUD who receive either methadone (opioid receptor agonist), extended-release naltrexone (antagonist) or buprenorphine (partial agonist).
Working with medicinal chemists and SARS-CoV-2 wildtype and variants in Wistar BSL-3 facilities, we aim to develop novel combination therapies against COVID-19 based on small molecules:
- Small molecules that amplify the natural interferon-based host resistance already shown in early therapy trials to reverse detrimental COVID-19 disease progression. We have found small molecules that enhance the natural antiviral responses mediated by existing type I interferons without inducing further inflammation damage on their own. Once developed, this novel therapy is also expected to be applicable for use with antivirals in future viral outbreaks.
- Small molecules that inhibit viral spread by directly blocking SARS-CoV-2 Spike protein (S) interactions with the ACE2 receptor and/or inhibiting the activity of the viral MProtease enzyme (Mpro or 3CLpro) in infected cells. We have identified small molecules that disrupt the interaction of the S protein with its human ACE2 receptor, thereby inhibiting viral entry; and small molecules that disrupt the MPro enzyme. MPro is a papain-like cysteine protease essential for processing the polyproteins that are translated from the viral RNA. Mpro can process at least 11 cleavage sites on the large polyprotein 1ab, the multifunctional protein involved in the transcription and replication of the viral RNAs. Inhibiting the activity of this enzyme would block viral replication. Because no human proteases with a similar cleavage specificity are known, such inhibitors are less likely to be toxic. We will also take advantage of the large and unique natural product and synthetic libraries available at Wistar to identify added lead molecules. We will validate identified hits using established SARS-CoV-2 Spike pseudo-virus systems as well as a live viral challenge model.
Development of these therapeutics, to be used in combination at onset of symptoms or in those at high risk of developing symptoms, is expected to limit viral infection, preserve lung tissue integrity, and prevent progression towards a cytokine release (“storm”) syndrome associated with mortality.
Myeloid cells are critical components of the tumor microenvironment. Under physiological conditions these cells are comprised of mature terminally differentiated cells: polymorphonuclear neutrophils (PMN) and other granulocytes; macrophages (MΦ); and dendritic cells (DCs). In cancer, the myeloid compartment is dramatically affected, which is now considered one of the major immunological hallmarks of cancer. Tumor-bearing (TB) hosts accumulate immunosuppressive MΦ, DCs that are ineffective in inducing potent immune responses. The prominent change in the myeloid compartment in cancer is the expansion of pathologically activated immature myeloid cells with a potent ability to suppress immune responses — myeloid-derived suppressor cells (MDSC). In TB mice, the total population of MDSC consists of three groups of cells: pathologically activated neutrophils (PMN-MDSC) are the most abundant (>75%); pathologically activated monocytes (M-MDSC) are less abundant (<20%); and early myeloid precursors represent a small (<5%) population. The current view considers changes in myeloid cells separately, with different mechanisms applied to the different cell types. The gap in our knowledge is how these different myeloid cells can interact with each other in TB hosts. We are investigating the bridge between different populations of myeloid cells in cancer and how they orchestrate their abnormal function. The ultimate goal of this project is not only to better understand the mechanism regulating myeloid cell function in cancer, but to develop novel approaches for regulation of immune responses in cancer.
According to National Cancer Institute (NCI) statistics, ovarian cancer represents 1% of all cancers, and more than 19,000 women are diagnosed every year in the U.S. An estimated one woman in 87 will develop ovarian cancer during her lifetime. Although many therapeutic approaches have been tested, including surgery, radiation, chemotherapy, and immunotherapy, ovarian cancer remains extremely difficult to treat, and novel therapeutic approaches are needed. This project, funded in part by the Department of Defense, is based on therapeutic strategies that can modulate myeloid cell apoptosis resulting in an increase of anti-tumor immune responses.
Absent direct clinical trials in humans, animal models of HIV infection are the best platform to explore novel pre-clinical anti-HIV strategies. Animal models for HIV infection include nonhuman primates and humanized mice. Humanized mice have emerged as a model able to be used for high-volume screening, yet the suboptimal immune differentiation that occurs has raised concern on the ability of this model to fully reflect all aspects of an immune response otherwise present in humans. The humanized mouse system has been developed to model HIV infection in humans, response to antiretroviral therapy (ART) and novel cure interventions, as well as study cancer immunotherapy in patient-derived xenograft models. A new WistarHu mice platform has been developed to support cancer immunotherapy and Wistar-based discovery of strategies against HIV based on assessing changes in viral measures on ART or effects on viral load rebound after ART interruption (Analytical Therapy Interruption, ATI). In support of this new platform, we have established ART formulations, HIV infection, HIV suppression, and characterized changes on immune reconstitution, persistent HIV measures, microbial translocation after ART and during an ATI. This platform is currently applied toward discovery and collaborative work.
With long-standing commitment from Philadelphia FIGHT and the University of Pennsylvania along with the Robert I. Jacobs Fund of The Philadelphia Foundation, the HIV-1 Patient Partnership Program was established to provide clinical material for basic research and to sponsor the Jonathan Lax Memorial Lecture (also supported by Ken Nimblett in memory of his husband, Rusty Miller, and The Summerhill Trust, established by Martha Stengel Miller). Research with clinical material obtained from this program is focused on mechanisms of AIDS immunopathology. This collaborative link between our research team and over 6000 HIV-1 patients in the Philadelphia region led to the largest HIV Cure clinical trial to date — the BEAT-HIV Study from 2014-2018.
The HIV-1 patient-partnership program involving participants in research is based at Philadelphia FIGHT (a community-based HIV-1 primary care provider) and Prevention Point Philadelphia. Our partnership with each community-based organization strives to develop trusted relationships and maintain meaningful, bi-directional lines of communication between investigators and communities most affected by HIV and substance use disorder. The primary objective of our community engagement strategy is to ensure communities have a clear understanding of a) the research being implemented, whether HIV-cure directed or other HIV, COVID, or monkeypox (MPOX) research, b) the stage of the research (including setting realistic expectations around HIV cure science), and c) how interested individuals can participate in and support our research agenda.
Social Science was added to our HIV cure research agenda to enhance both our preclinical and community engagement efforts. The Social Sciences Initiative assesses the acceptability of HIV cure interventions under development and conducts empirical ethics research related to HIV cure. Working in close collaboration, BEAT-HIV investigators and community stakeholders have developed a robust agenda of educational activities, community-based projects, and basic/clinical research designed to ensure comprehensive understanding and to provide guidance on the ethical conduct of HIV cure-directed research.
Above all, the Montaner laboratory makes its research accountable to our study participants and other stakeholders through community advisory board (CAB) review and community representation on Data Safety Monitoring Boards for clinical trials when active. In addition, we provide community-focused research presentations at Philadelphia’s AIDS Education Month, the annual Lax Lecture, and other community events, so that community members and other interested individuals are informed about the outcomes of patient-supported research.
The Jonathan Lax Lecture honors the memory of Jonathan Lax, a businessman, inventor, teacher, and one of the best-known AIDS activists in Philadelphia’s community-based clinical research network, where he volunteered with many groups to try and speed the drug approval process. He left funds to start a clinic — today called the Jonathan Lax Center — that is now the largest provider of AIDS care in Philadelphia, independent of a patient’s ability to pay. The Lax Lecture is a public lecture held each year at The Wistar Institute, where leading international HIV scientists interact with local researchers, clinicians, and patient advocates. Previous speakers include Gates Foundation HIV Frontiers and Biotechnology Accelerator head Mike McCune, Partners in Health founder and Harvard professor Paul Farmer, Project Inform founder Martin Delaney, and 2008 Nobel Laureate Françoise Barré-Sinoussi. In 2021, the Lax Lecture celebrated its 25th year by honoring Anthony Fauci for his dedication to serving people living with HIV – the first person to receive this honor twice. The 2024 honoree is Nobel Laureate in Physiology or Medicine, Dr. Drew Weissman.
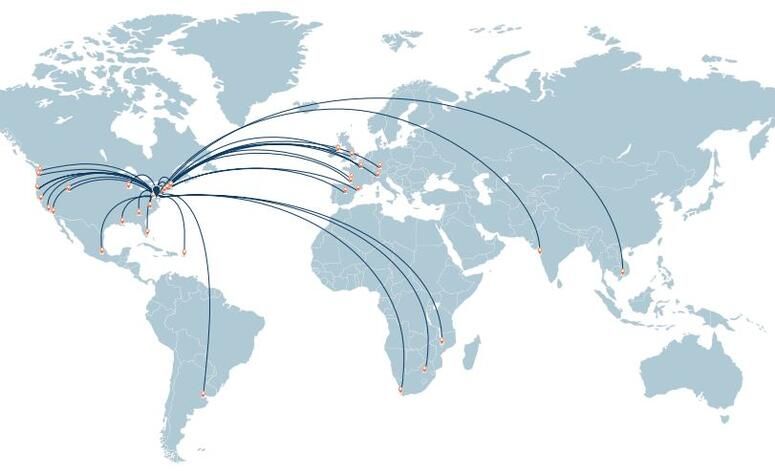
Global Health & Partnerships
The Wistar Institute fosters a local and global community that is unified by bold scientific thinking, leadership, and a collaborative spirit. Thought leaders from nonprofits, healthcare, pharmaceutical and biotechnology companies, governments and other agencies of influence choose to work collaboratively with Wistar scientists to accelerate the creation of new therapies for patients worldwide. We have connections in locations here.
-
Research Assistant Professor
Ian Tietjen, Ph.D.
-
Senior Staff Scientists
Livio Azzoni, M.D., Ph.D.
Emmanouil Papasavvas, Ph.D.
Costin Tomescu, Ph.D.
Zhe Yuan, Ph.D. -
Staff Scientist
Gwendolyn Cramer, Ph.D.
-
Postdoctoral Fellows
Avishek Buniya, Ph.D.
Khumoekae Richard, Ph.D.
Dmitry Zhigarev, Ph.D. -
Predoctoral Trainees
Ekaterina Shipilova
-
Research Assistants
Maria de Grecia Cauti Mendoza
Yong Chen
Matthew Fair
Adiana Ochoa Ortiz
Emery Register
Brian Ross
Paridhima Sharma
Qixun Sun
Xiao Sun
Guorui Zu -
Clinical Coordinator
Ken Lynn, R.N.
-
Clinical Research Assistant
Bashir Fadulalmola
Ola Mohamed -
BSL-2+ Laboratory Manager
Jessicamarie Morris
-
Associate Director, HIV Program
Beth Peterson
-
Administrative Coordinator
Chelsea Anderson
-
Postdoctoral fellow positions are available in the Montaner laboratory, with a focus on cancer and immunotherapy system-based research through a combination of both murine-based models and human translational studies. The preferred candidate is a recent Ph.D. or equivalent with a strong background in immunology, animal models, and molecular/cellular biology. Experience with molecular screening, bioinformatics, microscopy, and flow cytometry is a plus. Individuals eligible for National Research Service Award (NRSA) funding are highly desirable. Motivated candidates are encouraged to submit their CV and references to: montanerlab@wistar.org.
Staff Highlight: Ian Tietjen, Ph.D., Focuses on Bringing Traditional Medicine into Modern Research

Ian Tietjen, Ph.D., focuses on mechanisms of viral pathogenesis, and on drug discovery and development. He uses cell biology, genetics, and high-throughput chemical screening techniques to investigate the molecular properties of HIV reservoirs in addition to influenza, coronavirus, and nipah virus.
Tietjen collaborates with local communities, medicinal plant healers, and other knowledge keepers to sustainably and ethically document and determine the bioactivities of traditional medicines used in Southern Africa, Canada, and elsewhere.
Tietjen joined Wistar as a research assistant professor in the HIV Research Program in January 2020 and he is the head of the Small Molecule Discovery and Pharmacognosy Group. He was previously an assistant professor in the Faculty of Health Sciences in Vancouver, Canada, and has worked as a group leader in Molecular and Cellular Biology at Cardiome Pharma Corp. and a senior scientist at Xenon Pharmaceuticals.
SMALL MOLECULE DISCOVERY AND PHARMACOGNOSY GROUP
The Small Molecule Discovery and Pharmacognosy Group works with researchers, traditional healers, and other knowledge keepers who are interested in identifying and elucidating the molecular and biomedical properties of naturally produced chemical compounds and medicinal plants. The group primarily focuses on potential therapies for HIV, coronaviruses, influenza, and other infectious pathogens but also supports studies for cancer, metabolic diseases, and other illnesses. We provide assay development, laboratory training and instruction, and community engagement expertise to meaningfully work with local and Indigenous communities with traditional medicinal knowledge.
Individuals interested in working with the Small Molecule Discovery and Pharmacognosy Group can contact Ian Tietjen for more information at itietjen@wistar.org.
The mission of the BEAT-HIV Collaboratory is the define the most effective way to combine immunotherapy regimens to cure HIV.
Interview with Dr. Luis Montaner
The inaugural episode of The Wistar Institute’s podcast takes a deep dive with Dr. Luis Montaner, one of Wistar’s longest-serving scientists, as he discusses his lab’s groundbreaking HIV cure research and how he decided to dedicate his life to the cause of searching for an HIV cure.
Montaner Lab in the News
Selected Publications
Intact HIV reservoir estimated by the intact proviral DNA assay correlates with levels of total and integrated DNA in the blood during suppressive antiretroviral therapy.
Papasavvas, E., Azzoni, L, Ross, B.N., Fair, M, Yuan, Z., Gyampoh, K., Sciorillo, A.C., Paggliuzza, A., Lada, S., Wu, Gioxin, G., Goh, S.L., Banck-Teets, C., Holder, D.J., Zuck, P.D., Damra, M., Lynn, K.M., Tebas, P., Mounzer, K., Kostman, J.R., Abdel-Mohsen, M., Richman, D., Chomont, N., Howell, B.J., Montaner, L.J. 2021. “Intact HIV reservoir estimated by the intact proviral DNA assay correlates with levels of total and integrated DNA in the blood during suppressive antiretroviral therapy.” Clin. Infect. Dis. 72(3): 495-498. DOI: 10.1093/cid/ciaa809. PMID: 33527127.
Identification of the predominant human NK cell effector subset mediating ADCC against HIV-infected targets coated with BNAbs or plasma from PLWH
Tomescu, C., Kroll, K, Colon, K., Papasavvas, E., Frank, I., Tebas, P., Mounzer, K., Reeves, R.K., Montaner, L.J. 2021. “Identification of the Predominant NK Effector Subsets Mediating ADCC Against HIV-infected Targets Coated with BNAbs or Plasma from PLWH.” Eur J Immunol. 51(8): 2051-2061. DOI: 10.1002/eji.202149188. PMID: 34086344.
The Natural Stilbenoid (-)-Hopeaphenol Inhibits HIV Transcription by Targeting Both PKC and NF-κB Signaling and Cyclin-Dependent Kinase 9
Tietjen, I., Schonhofer, C., Sciorillo, A., Naidu, M.E., Haq, Z., Kannan, T., Kossenkov, A.V., Rivera-Ortiz, J., Mounzer, K., Hart, C., Gyampoh, K., Yuan. Z., Beattie, K.D., Rali, T., Shuda McGuire, K., Davis, R.A., Montaner, L.J. 2023. “The natural stilbenoid (-)-hopeaphenol inhibits HIV transcription by targeting both PKC- and NK-kappaB-signaling and cyclin-dependent kinase 9.” Antimicrobial Agents and Chemotherapy. 67(4): e01600-22. DOI: 10.1128/aac.01600-22. PMID: 36975214.
Susceptibility to 3BNC117 and 10-1074 in ART suppressed chronically infected persons
Tebas, P., Lynn, K.M., Azzoni, L., Cocchella, G., Papasavvas, E., Fair, M., Reeves, J.D., Petropoulos, C., Lalley-Chareczko, L., Kostman, J.R., Short, W.R., Mounzer, K., Montaner, L.J. 2023. “Susceptibility to 3BNC117 and 10-1074 in ART suppressed chronically infected persons.” AIDS. DOI: 10.1097/QAD.0000000000003575. PMID: 37070542.
Community engagement group model in basic and biomedical research: lessons learned from the BEAT-HIV Delaney Collaboratory towards an HIV-1 cure
Dubé, K., Peterson, B., Jones, N.L., Onorato, A., Carter, W.B., Dannaway, C., Johnson, S., Hayes, R., Hill, M., Maddox, R., Riley, J.L., Shull, J., Metzger, D., Montaner, L.J. 2023. “Community Engagement Group Model in Basic and Biomedical Research: Lessons Learned from the BEAT-HIV Delaney Collaboratory Towards an HIV-1 Cure.” Research Involvement & Engagement 2023; 9(39): 1 – 17. 9(1): 39. DOI: 10.1186/s40900-023-00449-y. PMID 37291622.



