Wistar Science Highlights: Zika Infection, Cancer Immunology and Immunotherapy
In a study published online in Nature, the lab of Dmitry I. Gabrilovich, M.D., Ph.D., Christopher M. Davis Professor and leader of the Immunology, Microenvironment and Metastasis Program, identified a critical step that happens when parts of the immune system switch sides and work for the tumor.
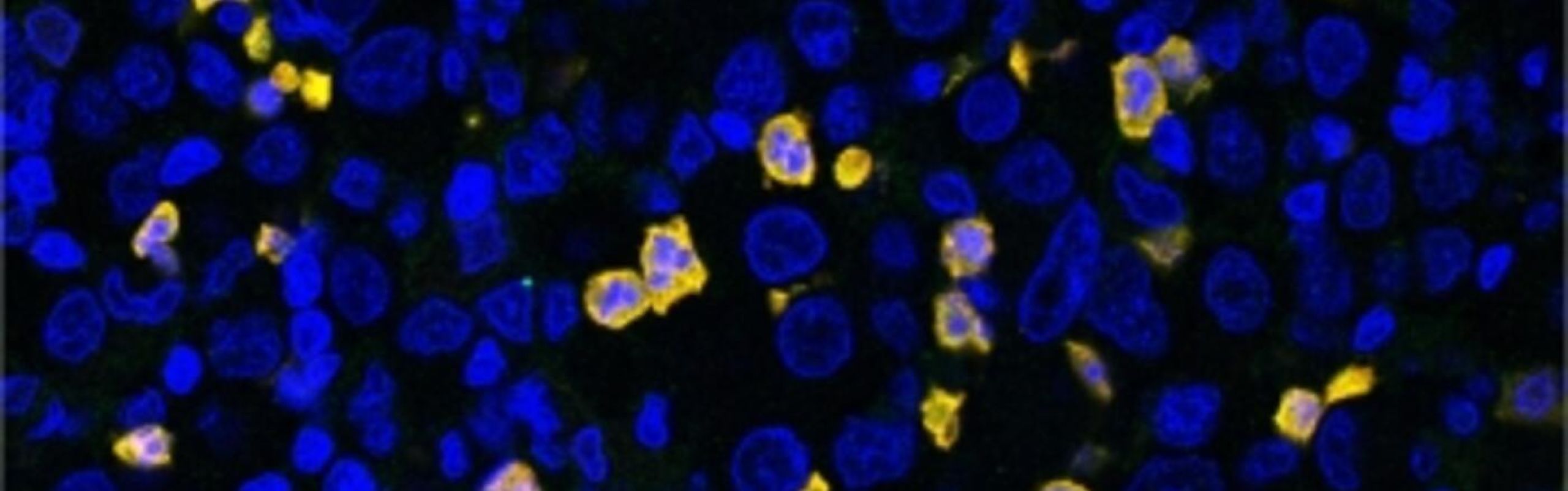
Myeloid derived suppressive cells (MDSCs) are a bone marrow-derived cell population that becomes pathologically activated in cancer patients and hinders the body’s antitumor immune response. They are associated with poor prognosis and resistance to immunotherapy.
“We need to better define how these cells work and what pathways are important, so that we can devise strategies to keep them at bay,” said Gabrilovich. “Our study represents an important step forward in that direction.”
They found that a gene that encodes for a protein called FATP2 is expressed at much higher levels in MDSCs isolated from tumor-bearing mice than in the corresponding cell population derived from healthy mice. They also described how FATP2 converts normal neutrophils into immunosuppressive cells in the presence of a tumor.
This study opens new opportunities for specific therapeutic targeting of MDSCs to inhibit tumor growth and enhance the anticancer activity of other immunotherapies. READ MORE
David B. Weiner, Ph.D., executive vice president and director of Wistar’s Vaccine & Immunotherapy Center, and W.W. Smith Charitable Trust Professor in Cancer Research, and his collaborators described a new approach for delivery of DNA-encoded monoclonal antibodies (DMAbs) for protection against Zika virus (ZIKV) infection.
In this study, published in Molecular Therapy, the lab engineered a synthetic DNA plasmid encoding a previously identified potent anti-ZIKV monoclonal antibody. When injected intramuscularly in mice and non-human primates, these DMAbs resulted in antibody presence in circulating blood for several weeks to months and provided long-term protection against infection in both small animals and, for the first time, non-human primate preclinical models.
“These properties, coupled with the ease of production and storage, support further development of DMAbs as a possibly ideal approach during infectious disease outbreaks to provide rapid protection to at-risk individuals and, in the case of Zika, to their offspring as well,” said Weiner. READ MORE
A study by researchers in Wistar’s Vaccine & Immunotherapy Center, published online in JCI Insights, led to the creation of a novel synthetic DNA approach for patient-specific production of cancer-targeting molecules called bispecific T cell engagers (BiTEs).
BiTEs are a type of artificial monoclonal antibody that directly binds to tumor targets, while at the same time binding to and activating killer T cells. In doing so, BiTEs act as a bridge between killer T cells and tumor cells, making sure that T cells see and specifically kill tumors with high accuracy.
BiTE production is difficult and they have a short duration in the bloodstream, requiring patients to be continuously infused for up to seven days for the treatment to be effective.
Teams led by Weiner and Kar Muthumani, Ph.D., assistant professor in the Vaccine & Immunotherapy Center, developed DNA-encoded bispecific T cell engagers (dBiTEs) that, when injected into the muscle in mouse models, provided the genetic instruction for muscle cells to make and launch the novel molecule directly into the bloodstream to seek and destroy tumors.
They designed dBiTEs specific for HER2, a cancer antigen found on breast and ovarian cancer cells, and tested them in mouse models of ovarian cancer. HER2 dBiTEs were highly expressed after just a single injection and were able to attract T cells that were activated to kill the tumor cells. Importantly, the tumor-killing activity persisted for more than a month.
“These results showed that dBiTEs are much more potent than traditional monoclonal antibodies. Not only did treatment extend survival of tumor-bearing mice, but also 80% of the animals treated with dBiTE were cured – a high bar to reach in this animal model,” said Muthumani. “Our results show that further exploration of the dBiTE approach for therapeutic development is warranted.” READ MORE