The Wistar Institute
Powering Cancer Research
Wistar’s scientific accomplishments could not be achieved without the support of our donors who recognize our research strengths, share our vision and are committed to tackling disease, improving human health and answering the most pressing scientif…
Our Educational Partnership with Leiden University Medical Center
Throughout its history, Wistar has successfully cultivated strategic relationships to accelerate the development of its discoveries toward the clinic. Recognizing that the potential of scientific discovery in biomedical research extends well beyond…
Research Collaborations in the Montaner Lab, Progress Toward an HIV Cure
Dr. Luis Montaner is a transformative leader in HIV research. Throughout his tenure at Wistar, his discoveries to find an HIV cure have elevated both the prestige and scientific prowess of the Institute while most importantly, helped inform the trea…
Learning How to Read the Book of Life
Research in the Gene Expression & Regulation Program at Wistar continues to reveal new knowledge on RNA and its functions to regulate how our genes are expressed and how that can go awry. In high school biology, we learned that our genes are the…
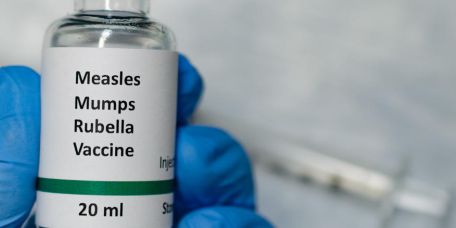
Combining Expertise, The Wistar Institute and Batavia Biosciences Partner to Expand Rubella Vaccine Manufacturing Around the World
Companies that make rubella vaccines have been getting harder and harder to come by. The global production of the rubella vaccine, supplied in combination with measles, mumps and varicella vaccines, which is the typical route for…
Wistar and Penn Medicine Awarded $11.7 Million Melanoma Research Grant from the National Cancer Institute
PHILADELPHIA — (Sept. 22, 2021) —The Wistar Institute and Penn Medicine have been awarded a prestigious $11.7 million Specialized Programs of Research Excellence, or SPORE, grant from the National Cancer Institute. The five-year award will fund thre…
Wistar Scientists Identify New Therapeutic Target in Ovarian Cancer Subtype With Poor Prognosis
PHILADELPHIA — (Sept. 21, 2021) — Mutations in the ARID1A gene are present in more than 50% of ovarian clear cell carcinomas (OCCC), for which effective treatments are lacking. Scientists at The Wistar Institute discovered that loss of ARID1A functi…
Dr. Noam Auslander: New Faculty Addition Brings Artificial Intelligence Research to Wistar
Wistar welcomes Dr. Noam Auslander as an assistant professor in the Cancer Center’s Molecular & Cellular Oncogenesis Program. She applies artificial intelligence (AI) through high-throughput computer approaches to interpret the very large sets o…
Dr. Hildegund Ertl: Vaccine Thought Leader and Formidable Presence
Dr. Hildegund Ertl, Wistar immunologist and vaccine developer, has been featured in local and national media and sought after by journalists as a source of expert opinions. A well-spoken scientist, always available to help unravel the many questions…
Bringing Together Cutting Edge Technologies, Daniel Claiborne, Ph.D., Charts a Course for Solving Puzzles About HIV Infection
Mice differ from people in many ways. But over the last decade, scientists have succeeded in engineering mice that are more humanlike in their ability to be infected with HIV, making it possible to study the disease, and develop vaccines and therapi…